November has been a busy month for Covid-19 in the United States.
With vaccine trial results released, there seems to be light at the end of the tunnel.
Boasting efficacy rates of 95% and 94.5%, respectively, Pfizer and Moderna’s vaccine results appear to promise the vast majority safety from the disease that has plagued our society.
Pfizer, a biopharmaceutical company, and Moderna, a similarly functioning biotechnology company, have produced effective vaccines and medicines to combat other viruses in the past.
However, the public must understand what an effective vaccine means.
Effective vaccines do not prevent infection.
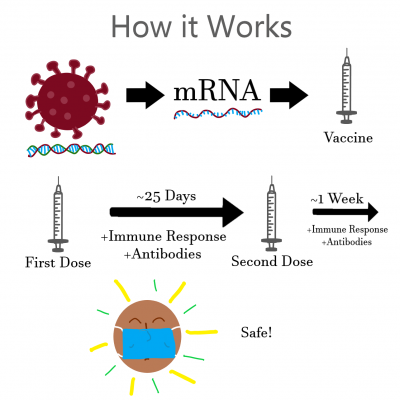
According to Pfizer, the effectiveness of a vaccine is how well it prevents the disease from becoming symptomatic through generating an immune response before the disease can take hold of your system.
Graphic courtesy of Annie Ahern
This is the goal with the COVID-19 vaccinations as well.
If an immune response given by a vaccine dose could generate antibodies against the infection without causing potentially life-threatening symptoms, that would be an effective vaccine.
Moderna filed for emergency FDA approval of their vaccine this month.
But what does this mean for us as CLC prepares for another semester in quarantine?
Essentially, nothing substantial for now. Dr. Fauci predicts the vaccine will not be distributed to the public until early spring.
The New York Times details how priority groups like healthcare workers and the elderly are the preferred choice for first vaccine doses as their vulnerability, exposure level and death rate all exceed those of the average citizen.
For at-risk groups such as medical workers and residents of live-in care facilities, there have been mentions1 of early distribution.
According to the Chicago Tribune, Illinois plans to remain in accordance with these terms. However, with estimates given by the Illinois Department of Public Health Director ranging from 400,000 initial vaccine doses available down to less than 80,000 , it’s unclear how effective any distribution plan would be with Illinois’ current population standing at around 12 ½ million residents.
For those who do get vaccinated, freedom from the constraints of COVID protocols will not be immediate.
Both the Moderna and Pfizer vaccines are mRNA vaccines, meaning they use a bite of genetic information from the virus to develop an immune response.
Pfizer explained that immune response, a smaller non-symptomatic infection of the virus, develops antibodies that prevent the patient from succumbing to the illness later on.
The catch here is that mRNA viruses are not instant and require two doses spread an average of 25 days apart and then one more week to build sufficient immunity. This means even if you receive a vaccine, you are not safe from contracting COVID-19 for a significant amount of time.
Additionally, until all members of the population are vaccinated, there is still the possibility of transmission that would require covid protocols to remain in place for long past the first batch of vaccinations.
Distribution and production of the vaccines is also a major concern.
While they are similar companies, Pfizer and Moderna differ significantly in the paths they took to create their effective vaccines.
Pfizer did not accept any money from government institutions, and so they essentially owe nothing to those institutions in the way of reliable and reasonable pricing.
However, in a surprising turn of events, they are charging only slightly more than Moderna (who was institutionally funded) for their two-dose system of vaccination.
According to Forbes and CNBC, the approximate cost of a two-dose system comes to $40, which the US Government is currently paying happily.
In comparison, Moderna’s vaccine is charging a mere $15 per dose ($30 per two-dose system), only slightly lower than Pfizer. `
Graphic courtesy of Easton Herbon
This is, however, without adding in the $900 million the National Institute of Health has given Moderna for research.
According to Moderna, it’s also worth noting that they will not maintain that lower pricing for individuals or private citizens.
However, with the immense amount of technology available and the billions of customers and consequently, billions of dollars promised to every company that produces a vaccine, there are surely to be more competitors on the market soon.
For example, Johnson and Johnson is currently in phase three of their vaccine trial; both Pfizer and Moderna released their vaccines for emergency use authorization following stage three, which is a singular dose given once.
There truly is light at the end of the tunnel, but it is always encouraged to stay informed and stay safe.